PlaqueIQ™, based on CT Virtual Histology™
Enabling patient-specific treatment pathways with PlaqueIQ.
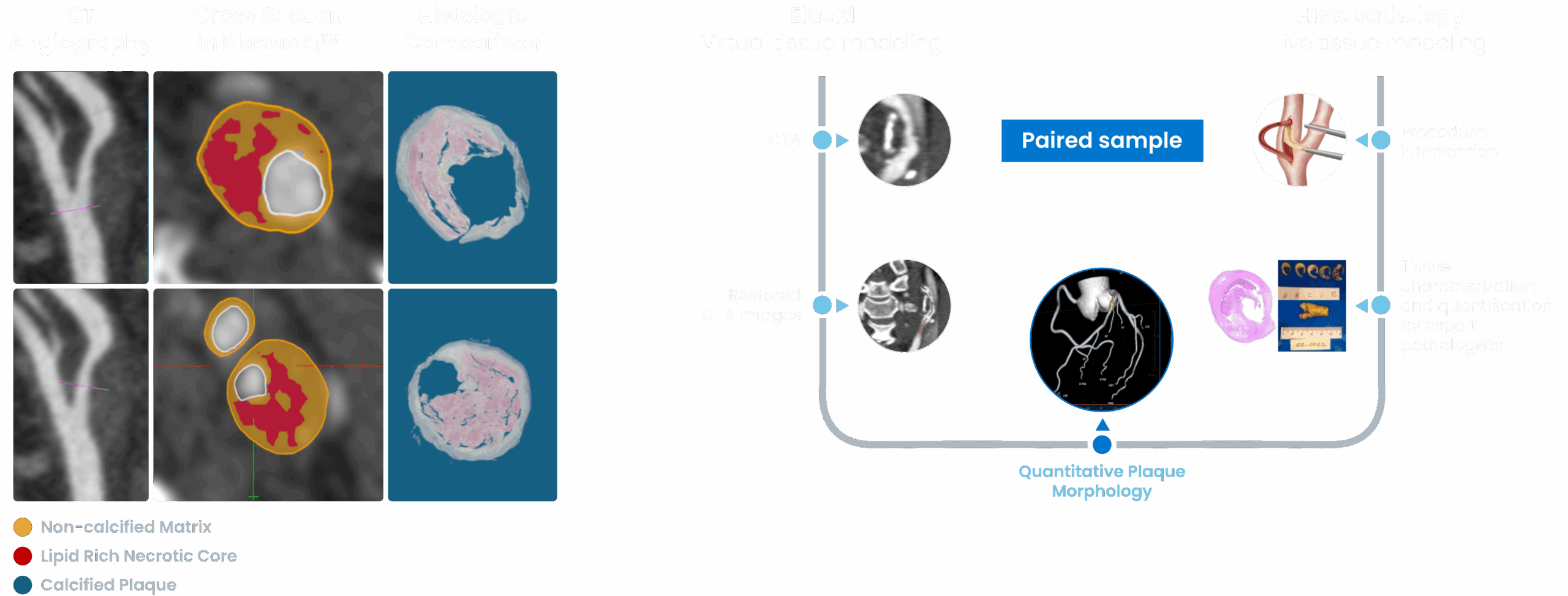
PlaqueIQ is trained and validated on ground-truth histology, the gold standard for plaque characterization.
Plaque detection is just the beginning.
The only CTA-based plaque assessment powered by ground-truth histology
Developed in partnership with world-renowned cardiovascular pathologists, ground truth histology serves as the basis for training Elucid’s powerful algorithm to objectively quantify vessel structure and plaque composition.
Clinical Problem
About half of Americans between ages 45 and 84 have atherosclerosis and don’t know it.¹
Despite advances in therapeutics, CVD remains the most common cause of death in the world. Moreover, WHO estimates 80 to 90% of heart attacks as preventable. We believe that current diagnostics miss key information on the type of plaque patients have in their arteries.
While physicians look at many risk factors to evaluate patient risk, such as age, diet, and lifestyle, the strongest predictor of future events is the amount and type of plaque patients have in their arteries.²

1. Hafiane, A. Vulnerable plaque, characteristics, detection, and potential therapies, J. Cardiovasc. Dev. Dis. 6 (3) (2019). Available from: https://www.mdpi.com/2308-3425/6/3/26.
2. Pahwa R, Jialal I. Atherosclerosis. [Updated 2023 Aug 8]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK507799/ (accessed 03.17.2023)
Providing physicians with comprehensive patient, vessel, and lesion-level plaque composition and quantification data.
Solution
PlaqueIQ™ is the first and only FDA-cleared, non-invasive plaque analysis based on objective histology rather than subjective CCTA visual estimates. PlaqueIQ quantifies and classifies plaque morphology based on ground-truth histology, the gold standard for characterization of plaques.
Our plaque analysis software offers the potential to provide greater insights into plaque composition when compared to traditional Hounsfield Unit thresholding. PlaqueIQ can provide physicians with the ability to better determine medical management and help visualize and plan their interventional decisions.
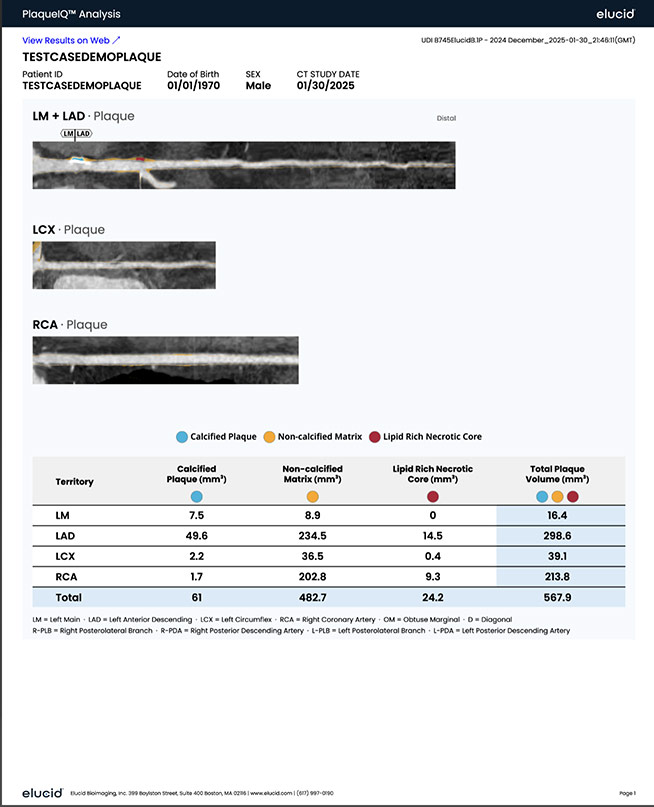
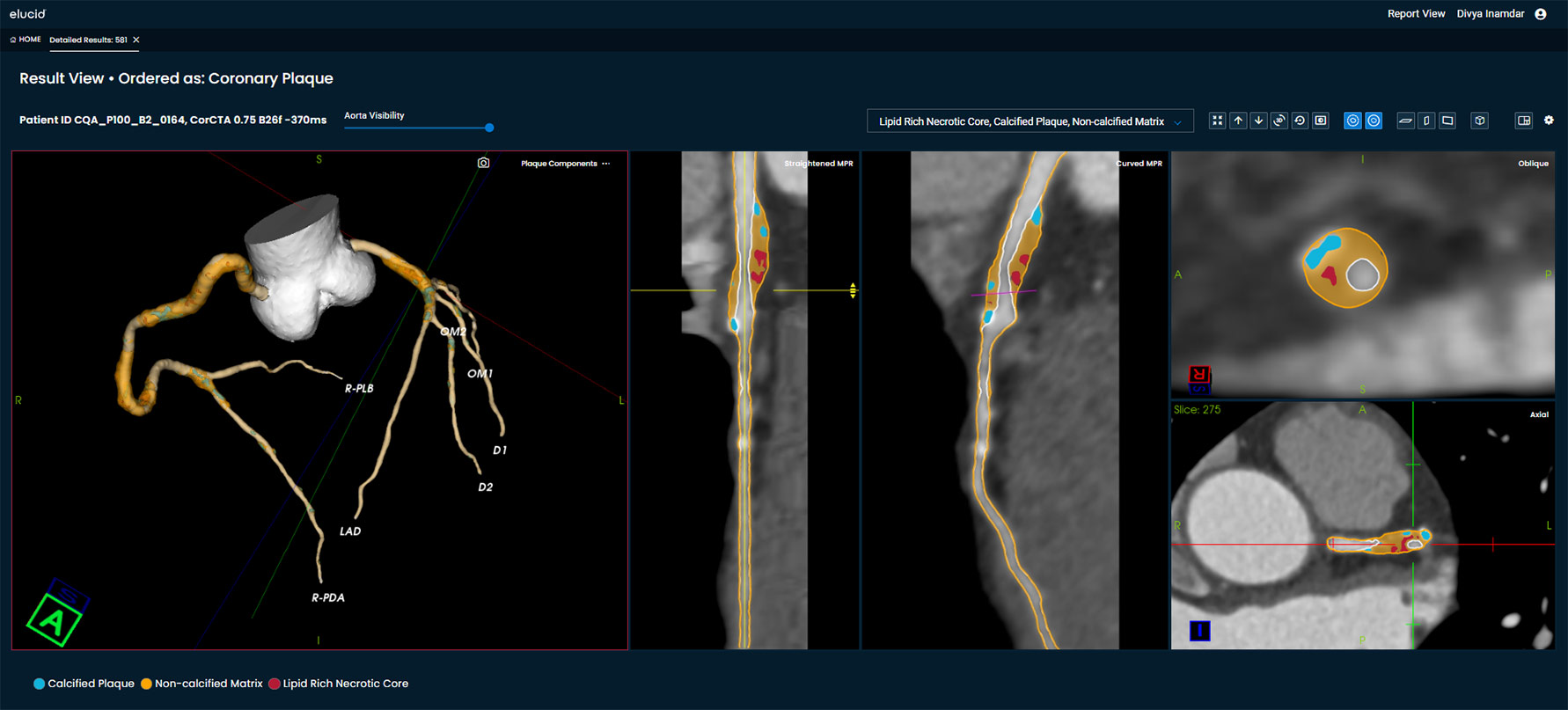
Our plaque analysis with an actionable report includes 3D visualization of vessel structure, critical arterial volumes, and plaque quantification including lipid rich necrotic core, calcified, and non-calcified components.
Elucid, via PlaqueIQ and FFRCT (under development), offers physicians CT Virtual Histology™, where physicians can understand the plaque tissue histology of a patient with a non-invasive test.
Histology Validation
Elucid’s PlaqueIQ algorithms were validated on histopathologic specimens, including calculations of anatomic structure and calculations of tissue characteristics. The histopathologic validation demonstrated the following performance:
| Plaque Type |
Elucid’s PlaqueIQ |
| Total Plaque | r = 0.99 |
| Calcified | r = 0.95 |
| Non-Calcified | r = 0.87 |
| LRNC/LAP | r = 0.89 |
*LRNC Area includes areas of LRNC and Intra-Plaque Hemorrhage as identified by pathologists
Elucid PlaqueIQ™
- Tailor treatment to patient-specific vasculature
- Utilize actionable, risk-based, lesion-specific plaque composition
- Support clinical decisions such as intervention versus drug-therapy
- Supports physician understanding of patient risk to tailor medical guidance and prevent heart attack and stroke
- Visualize the plaque type and amount in a patient’s vessels, to support physician assessment of stable plaque versus more high-risk plaque
- Supports physician determination of the most appropriate therapy based on the patient’s unique plaque composition
- If you are interested in submitting a proposal for a research study, please click here
PlaqueIQ™ Technology
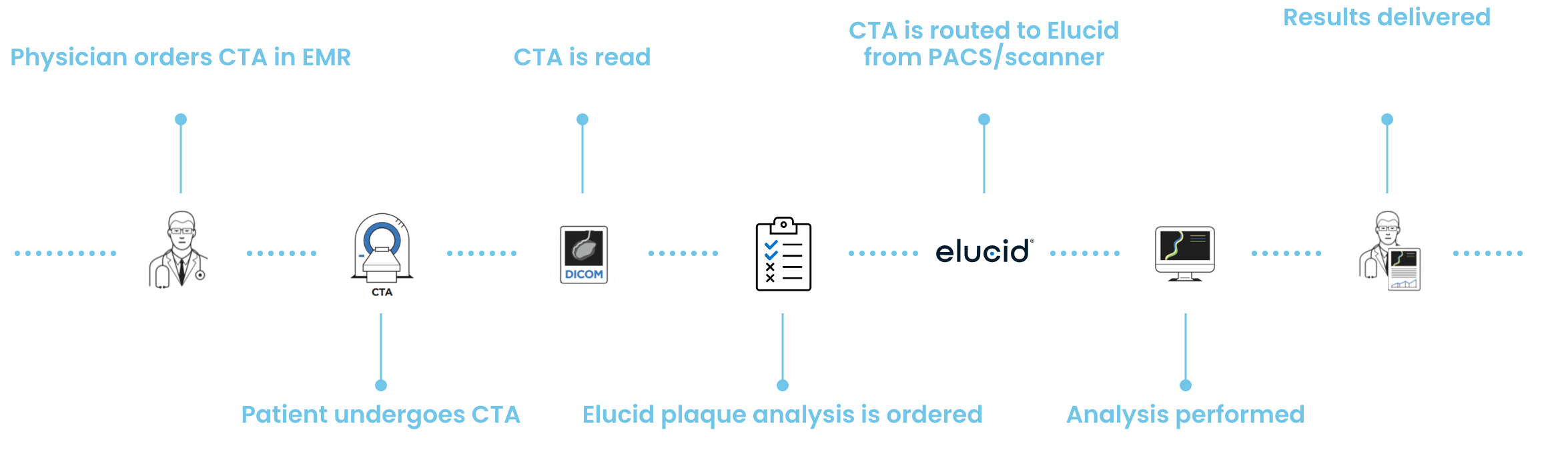
PlaqueIQ Results are Delivered to Physicians in Two Ways
Visualize lesion-level plaque composition, including Calcified Plaque, Non-Calcified Matrix, and Lipid Rich Necrotic Core
The Elucid algorithms demonstrate excellent correlation and are highly accurate for tissue characterization when compared to ex vivo histopathology.”
Secure, Workflow-Oriented Technology
News
Elucid Announces Broad Reimbursement for Quantitative Coronary Plaque Analysis
Effective January 1, 2026, its Plaque-IQ™ coronary plaque analysis has received a new Category I Current Procedural Terminology (CPT®) designation, alongside increasingly widespread coverage and reimbursement from both public and private payors. Together, these developments underscore the clinical value of coronary plaque analysis and establish favorable conditions for broader adoption of the technology.
Elucid Gains Broad CMS Reimbursement of Coronary Plaque Analysis Using PlaqueIQ™
Elucid has announced new Hospital Outpatient Prospective Payment (OPPS) and Physician Fee Schedule (PFS) decisions from the U.S. Centers for Medicare & Medicaid Services (CMS) that establish significant reimbursement for coronary plaque analysis using PlaqueIQ across care settings.
Elucid Launches PlaqueIQ Image Analysis Software for the Carotid Arteries
The first and only CT-based plaque analysis indicated for the carotid anatomy that can help physicians assess risk of rupture and ischemic stroke 1 BOSTON - October 29, 2025 – Elucid, an AI medical technology company focused on providing physicians with a more precise...
About
Plaque detection is just the beginning.
PlaqueIQ™ is trained and validated on ground-truth histology, the gold standard for plaque characterization, providing physicians with comprehensive patient, vessel, and lesion-level plaque composition and quantification data.
Our Portfolio
Elucid PlaqueIQ™
The only FDA-cleared, non-invasive CTA plaque assessment that’s powered by ground-truth histology.
Elucid FFRCT
Leveraging histology-based plaque composition to calculate lesion-level FFRCT measurements. Coming soon!
Elucid Clinical Research
Use Elucid’s CTA analysis capabilities for potential research on events tracking, pharma research and more.
Elucid Leadership

Scott Huennekens
Chairman of the Board

Kelly Huang, PhD
President & CEO

Blake Richards
Chief Operating Officer

Andrew Miller
Chief Technology Officer & SVP, Engineering

Windi Hary
Chief Regulatory & Quality Officer

Sophie Khem
SVP, Clinical Quality & Delivery

Amy Kruglak
SVP, People & Leadership Development

Kevin Mathews
SVP, Marketing

Amir Ahmadi, MD
Lead Scientific Advisor
Contact
Contact us today to learn more about Elucid or to schedule a product demo.
Boston, MA 02116